Disentangling the heterogeneity of multiple sclerosis through identification of independent neuropathological dimensions
Alyse de Boer, Aletta M. R. van den Bosch, Nienke J. Mekkes, Nina L. Fransen, Ekaterina Dagkesamanskaia, Eric Hoekstra, Jörg Hamann, Joost Smolders, Inge Huitinga & Inge R. Holtman
Acta Neuropathologica 147, article number 90 (2024)
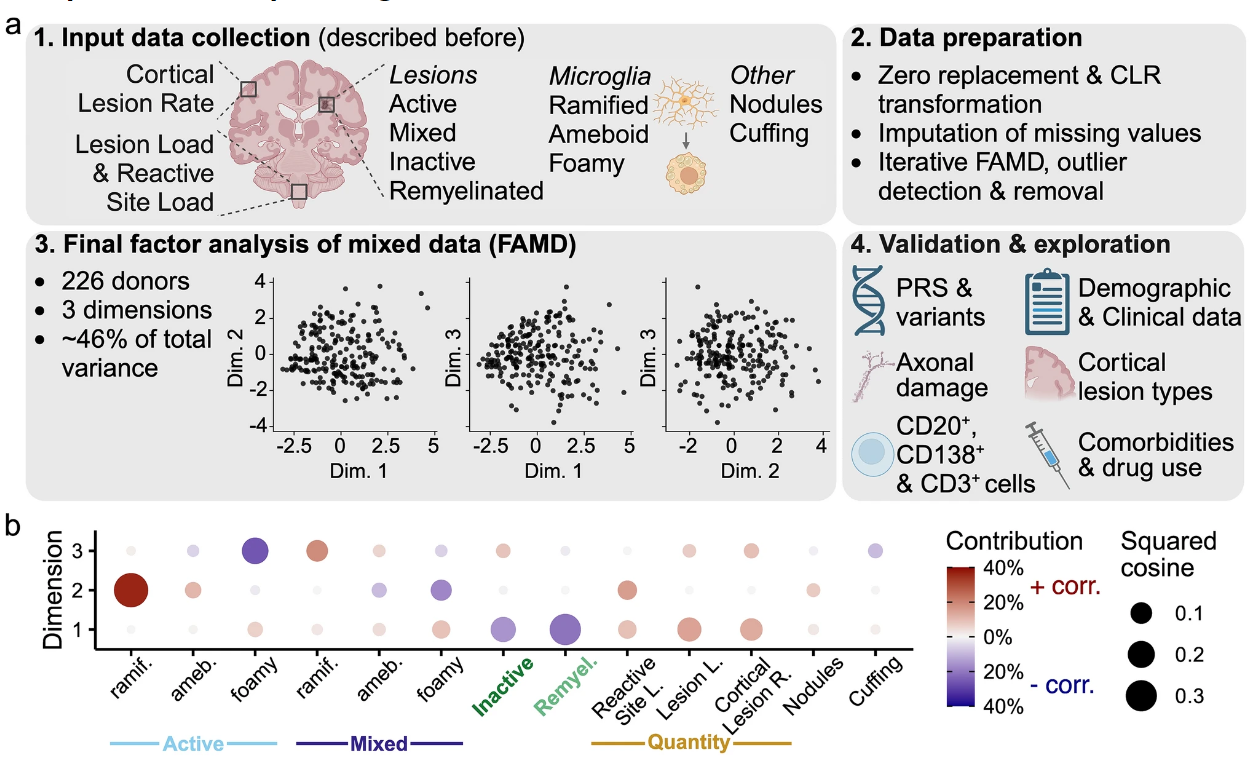
Multiple sclerosis (MS) is a heterogeneous neurological disorder with regards to clinical presentation and pathophysiology. Here, we investigated the heterogeneity of MS by performing an exploratory factor analysis on quantitative and qualitative neuropathology data collected for 226 MS donors in the Netherlands Brain Bank autopsy cohort. Three promising dimensions were identified and subsequently validated with clinical, neuropathological, and genetic data. Dimension 1 ranged from a predominance of remyelinated and inactive lesions to extensive pathological changes, higher proportions of active and mixed lesions, and foamy microglia morphology. This pattern was positively correlated with more severe disease, the presence of B and T cells, and neuroaxonal damage. Scoring high on dimension 2 was associated with active lesions, reactive sites, and the presence of nodules. These donors had less severe disease, a specific pattern of cortical lesions, and MS risk variants in the human leukocyte antigen region, the latter indicating a connection between disease onset and this neuropathological dimension. Donors scoring high on dimension 3 showed increased lesional pathology with relatively more mixed and inactive lesions and ramified microglia morphology. This pattern was associated with longer disease duration, subpial cortical lesions, less involvement of the adaptive immune system, and less axonal damage. Taken together, the three dimensions may represent (1) demyelination and immune cell activity associated with pathological and clinical progression, (2) microglia (re)activity and possibly lesion initiation, and (3) loss of lesion activity and scar formation. Our findings highlight that a thorough understanding of the interplay between multiple pathological characteristics is crucial to understand the heterogeneity of MS pathology, as well as its association with genetic predictors and disease outcomes. The scores of donors on the dimensions can serve as an important starting point for further disentanglement of MS heterogeneity and translation into observations and interventions in living cohorts with MS.
Read